Abstract
VOD/SOS is a potentially life-threatening complication of HCT. Historically, per Baltimore criteria, hyperbilirubinemia (bilirubin >2 mg/dL) has been a key criterion for VOD/SOS diagnosis, although recent guidelines also acknowledge VOD/SOS without elevated bilirubin. Recent studies have reported anicteric VOD/SOS (bilirubin ≤2 mg/dL at diagnosis) in >20% of patients. This post-hoc analysis of data from a large registry study in France presents patient characteristics and effectiveness and safety outcomes by bilirubin level at VOD/SOS diagnosis in patients treated for VOD/SOS post-HCT.
DEFIFrance is a post-marketing registry study that collected retrospective and prospective real-world data on patients receiving defibrotide at 53 HCT centers in France. Included in this post-hoc analysis are all patients treated with defibrotide for VOD/SOS post-HCT. Diagnosis of VOD/SOS was at the investigator's discretion using standard criteria, per typical clinical practice. Disease severity was categorized using adult EBMT severity criteria in patients aged ≥18 years, and patients aged <18 years were retrospectively/prospectively categorized using pediatric EBMT severity criteria. The primary endpoints were Kaplan-Meier (KM)-estimated Day 100 post-HCT survival and complete response (CR; total serum bilirubin <2 mg/dL and multiorgan failure resolution per investigators' assessment). Serious treatment-emergent adverse events (TEAEs) of interest were also collected: hemorrhage, coagulopathy, immunogenicity, injection-site reaction, septicemia, infection, and thromboembolic event, irrespective of relationship to treatment.
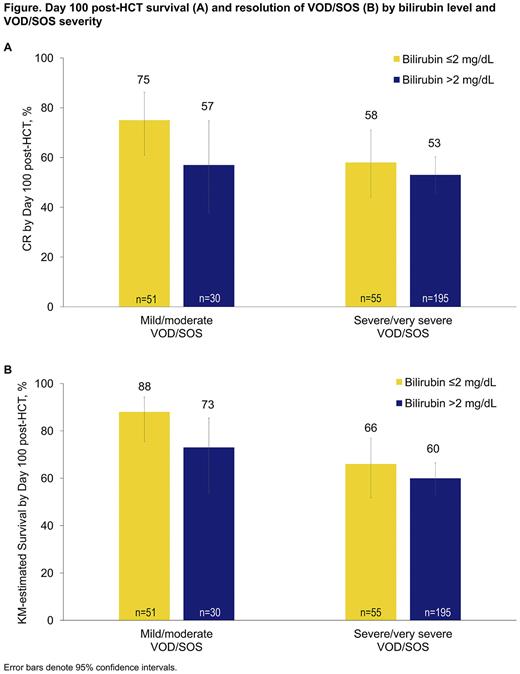
Of the 336 defibrotide-treated patients with VOD/SOS post-HCT, 109 (33%) had bilirubin ≤2 mg/dL, and 226 (67%) patients had bilirubin >2 mg/dL at diagnosis; 1 patient had missing data. Anicteric VOD/SOS was more common in pediatric patients (49% overall and 26% among patients with severe/very severe VOD/SOS) than adults (26% overall and 21% among patients with severe/very severe VOD/SOS), resulting in a lower median age in those with bilirubin ≤2 mg/dL (29.0 years; range: 0, 71) versus patients with bilirubin >2 mg/dL (47.5 years; range: 0, 74). The most common primary disease in each group, bilirubin ≤2 mg/dL and bilirubin >2 mg/dL, was AML (22% and 29%, respectively). Most patients in each group had no prior HCTs (91% and 88%, respectively) and underwent myeloablative conditioning (69% and 54%, respectively). Prior exposure to gemtuzumab or inotuzumab ozogamicin had occurred in 14% and 8% of patients with bilirubin ≤2 mg/dL and bilirubin >2 mg/dL, respectively. Rates of Day 100 survival post-HCT and resolution of VOD/SOS tended to be higher in patients with bilirubin ≤2 mg/dL versus patients with bilirubin >2 mg/dL (Figure). Outcomes in patients with mild/moderate VOD/SOS post-HCT were more favorable than in those with severe/very severe disease, regardless of bilirubin level at diagnosis.
Serious TEAEs of interest occurred in 25% of mild/moderate and 40% of severe/very severe patients with bilirubin ≤2 mg/dL; in patients with bilirubin >2 mg/dL, 33% and 26% of patients with mild/moderate and severe/very severe VOD/SOS had serious TEAEs of interest. Most common serious TEAEs of interest by category in each group, bilirubin ≤2 mg/dL and bilirubin >2 mg/dL, were hemorrhage (mild/moderate: 20%, severe/very severe: 24%, respectively; mild/moderate: 17%, severe/very severe: 14%, respectively) and infection (mild/moderate: 6%, severe/very severe, 24%, respectively; mild/moderate: 10%, severe/very severe: 15%, respectively).
In this post-hoc analysis, defibrotide-treated patients with anicteric VOD/SOS tended to have better outcomes than those with bilirubin >2 mg/dL at VOD/SOS diagnosis. Similarly, outcomes tended to be better in those treated at a less severe stage of disease. Taken together, these findings highlight the importance of prompt diagnosis and treatment of VOD/SOS without the requirement for elevated bilirubin, emphasizing the relevance of using VOD/SOS diagnostic criteria that do not require hyperbilirubinemia. The safety profile of defibrotide in the real-world setting was consistent with previous studies.
Disclosures
Duchêne:Jazz Pharmaceuticals: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Dronamraju:Jazz Pharmaceuticals: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Yakoub-Agha:Jazz Pharmaceuticals: Honoraria. Labopin:Jazz Pharmaceuticals: Honoraria. Dalle:Sanofi: Honoraria; Medac: Honoraria; Orchard: Honoraria; Teva: Current equity holder in private company; Vertex: Honoraria; Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria. Mohty:Celgene: Honoraria; Bristol Myers Squibb: Honoraria; Takeda: Honoraria; Amgen: Honoraria; Astellas: Honoraria; Novartis: Honoraria; Adaptive Biotechnologies: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria.
OffLabel Disclosure:
The abstract describes outcomes in pts with mild/moderate VOD/SOS, who are not included in the indicated population in the product label
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal